In Case You Missed It! Trichomoniasis Treatment Update 2021
By: Denise Machado, PharmD Candidate1; Lukagea Samedi, PharmD Candidate1; Elizabeth Sherman, PharmD, AAHIVP1,2,3
1. Nova Southeastern University, College of Pharmacy
2. Memorial Healthcare System
3. South Florida, Southeast AIDS Education and Training Center
On July 23rd, 2021, the Sexually Transmitted Infections Treatment Guidelines were updated by CDC to replace the previous 2015 guidelines.1,2 This update provides current evidence-based guidance for prevention strategies, diagnostic suggestions, and treatment recommendations of sexually transmitted infections in the US. A notable update was made to the management of Trichomonas vaginalis.
Trichomoniasis is a treatable, sexually transmitted infection caused by a protozoan parasite that infects the squamous epithelium of the genital tract.3 CDC states that up to 85% of people who contract T. vaginalis have minimal to no genital symptoms.1 However, symptoms may occur including prostatitis and epididymitis in men and malodorous, yellow- green vaginal discharge in women.
In 2018, there were approximately 6.9 million new trichomonas infections in the U.S and 156 million new cases world- wide in 2016, making it the most common non-viral STI.5,6 Although detection and treatment are important for reproductive health, trichomoniasis is not a reportable disease and surveillance is limited. Currently, the US prevalence for trichomoniasis among men and women are disparate, where women are four times as likely as men to present with an infection.4 Compared with non-Hispanic white females, T. vaginalis prevalence rates are significantly higher in Black/ African American females (0.8% vs 9.6%, respectively) and in Hispanic/Latina females (1.4%).5 According to a report published in the Journal of the American Sexually Transmitted Diseases Association, trichomoniasis prevalence in adolescents between 15 and 24 years of age comprised 15.6% of all infections.5 Since T. vaginalis facilitates the transmission of HIV, it is important to note that 36.4% of women with HIV will likely test positive for T. vaginalis.4
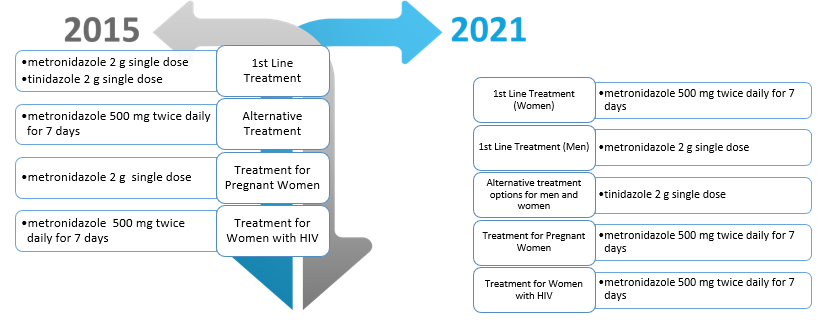
CDC STI guidelines for the treatment of trichomoniasis were updated in July 2021.1 The most significant changes made were that previously, first line recommended treatment for T. vaginalis was metronidazole 2 g orally in a single dose or tinidazole 2 g orally in a single dose for BOTH men and women. In the 2021 updates, first line recommended treatment is separated by sex, recommending that women adhere to a treatment course of metronidazole 500 mg orally twice daily for 7 days and that men adhere to metronidazole 2 g orally in a single dose. Figure 1 depicts other changes made to the trichomoniasis treatment recommendations, including alternative treatment and treatment among pregnant women. Although not listed in Figure 1, but of equal importance, recommendations were updated for recurrent infections due to reinfection or treatment failure. In women who have been reinfected and previously treated with metronidazole 500 mg orally twice daily for 7 days, a repeat treatment with the same regimen should be completed. However, if treatment failed without re-exposure, then she should be treated with metronidazole or tinidazole 2 g orally once daily for 7 days. If it is a man who is experiencing reinfection after completing a treatment course with metronidazole 2 g orally as a single dose, then he should repeat the same treatment regimen. However, if he was not reinfected, and the recurrence is due to treatment failure, then he should be treated with metronidazole 500 mg orally twice daily for 7 days.
The decision to adjust the first line treatment for trichomoniasis was based on a study published in The Lancet in 2018.7 This controlled trial randomly assigned 623 women with T. vaginalis infections to the single dose metronidazole 2 g orally regimen vs the 7-day metronidazole 500 mg orally twice daily regimen.7 The study, which ended early due to funding limitations, found that patients in the 7-day oral metronidazole treatment group had a lower probability of receiving a positive result at the 1- month test-of-cure versus the single dose group.7 Of the 623 women in the study, 34 of 312 (11%) in the 7-day treatment group and 58 of 311 (19%) in the single dose group were positive for T. vaginalis at test-to-cure (p<0.0001).7
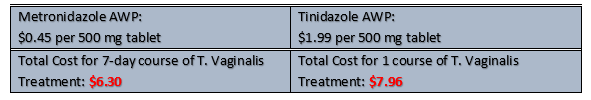
Displayed in Figure 2 is the average wholesale price cost comparison between metronidazole and tinidazole tablets. This figure illustrates that treatment options are both affordable and similar.
Figure 1. Comparison of T. vaginalis treatment recommendation: 2015 vs. 2021 CDC Guidelines
Figure 2. Average Wholesale Price (AWP) cost comparison between metronidazole and tinidazole treatment course
References:
- Workowski KA, Bachmann LH, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(No.RR4): 1187.doi:10.15585/mmwr.rr7004a1
- Workowski KA, Bolan GA, et al. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep. 2015;64(No. RR-3). PMID:26042815
- Kissinger, P. Trichomonas vaginalis: A Review of Epidemiologic, Clinical and Treatment Issues. BMC Infect Dis. 2015;15:307. doi:10.1186/s12879-015-1055-0
- Flagg EW, Meites E, et al. Prevalence of Trichomonas vaginalis Among Civilian, Noninstitutionalized Male and Female Population Aged 14 to 59 Years: United States, 2013 to 2016. Sex Transm Dis. 2019;46(10):e93-e96. doi:10.1097/OLQ.0000000000001013
- Kreisel KM, Spicknall IH, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sex Transm Dis. 2021; 48(4):208-214. doi:10.1097/OLQ.0000000000001355
- Rowley J, Vander- Hoorn S, et al. Chlamydia, Gonorrhea, Trichomoniasis and Syphilis: Global Prevalence and Incidence Estimates, 2016. Bull World Health Organ 2019;97: 548–562P. doi:10.2471/BLT.18.228486
- Kissinger P, Muzny CA, et al. Single-dose vs 7 Day-dose Metronidazole for the Treatment of Trichomoniasis in Women: An Open Label, Randomised Controlled Trial. Lancet Infect Dis. 2018;18(11):1251-1259. doi:10.1016/S1473-3099(18)30423-7