Extra! Extra! Read all about it! 2021 Guideline Updates for Treatment of Chlamydia & Uncomplicated Gonorrhea
By: Saba Farahmand, PharmD Candidate1; Faiza Mansuri, PharmD Candidate1; Sheila Montalvo, PharmD, BCPS2; Elizabeth Sherman, PharmD, AAHIVP1,2,3
- College of Pharmacy, Nova Southeastern University
- Memorial Healthcare System
- South Florida, Southeast AIDS Education and Training Center
Along with the occurrence of millions of sexually transmitted infections (STIs) each year, the use of antimicrobials is increasing worldwide leading to the development of resistance among individuals.1 In 2018, a systematic literature review was conducted by Centers for Disease Control and Prevention (CDC) to revise the 2015 STI guidelines. They analyzed the treatment for STIs, signs and symptoms and prevention of sequelae and transmission. On July 23, 2021, CDC updated STI guidelines including the treatment of chlamydia and uncomplicated gonorrhea to overcome resistance development. According to these updated guidelines, the recommended treatment of chlamydia is now doxycycline 100 mg twice daily orally for 7 days. Additionally, the treatment of uncomplicated gonorrhea is now a single dose of ceftriaxone 500 mg intramuscularly (IM).2 Antimicrobial stewardship is a program that promotes the proper use of antimicrobials to avoid the resistance which is one of the major public health problems in the United States. The new updates have been recommended based on the concerns regarding increased rates of azithromycin resistance and ceftriaxone resistance when used at lower doses.3
The Basics
Chlamydia is the most common STI in the United States with an estimate of 1.8 million cases in 2019.4 It can cause infections in the cervix, genitals, urethra, rectum, throat, and eye infections usually seen in infants during birth. Chlamydia is caused by Chlamydia trachomatis that can cause complications in both men and women such as pelvic inflammation and reactive arthritis.5
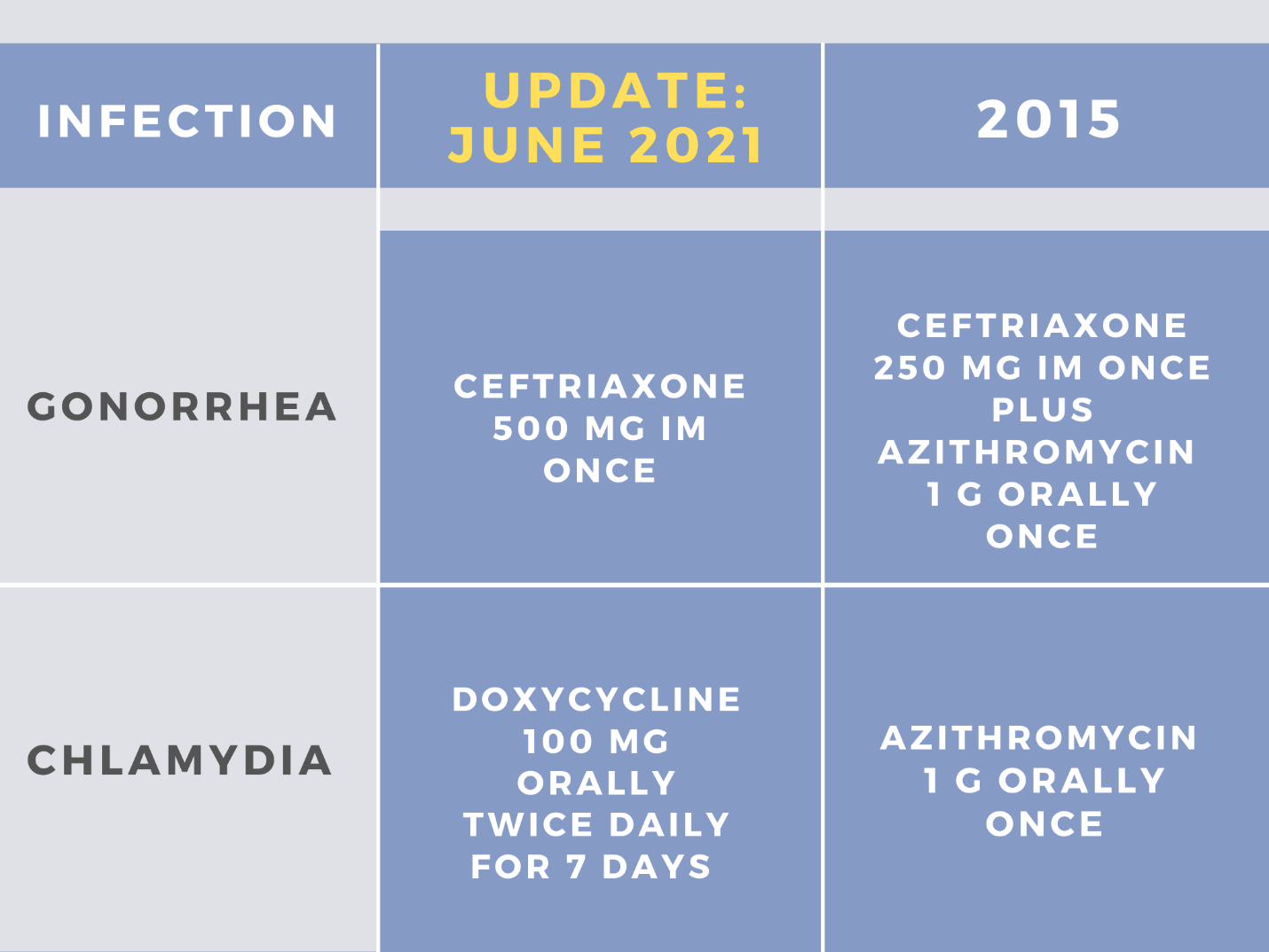
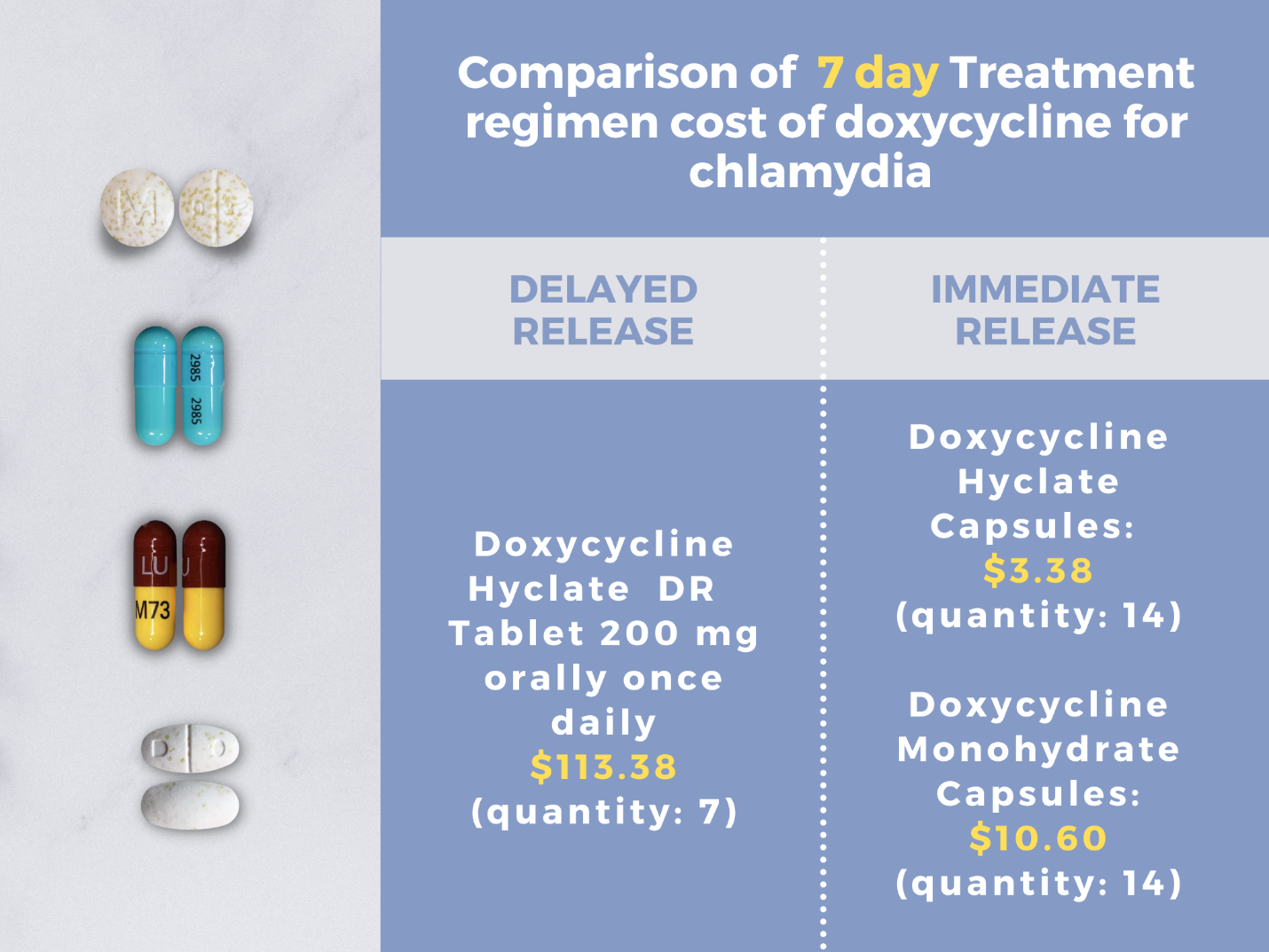
CDC STI guidelines were recently updated in July 2021. Prior to this, clinicians referred to treatment recommendations appearing in the 2015 guideline document. In 2015, the recommended regimen for chlamydia in adults and adolescents was azithromycin 1 g orally in a single dose or doxycycline 100 mg orally twice daily for 7 days. Per CDC’s new 2021 update, doxycycline 100 mg orally twice daily for 7 days is now the recommended treatment. Figure 1 depicts the changes in both chlamydia and gonorrhea treatment recommendations across the CDC STI 2021 and 2015 guidelines. Alternative regimens include either azithromycin 1 g orally in a single dose or levofloxacin 500 mg orally once daily for 7 days. Notably, there is a delayed release (DR) formulation of doxycycline available on the market to lighten the pill burden for patients and to ease gastrointestinal effects.2 This regimen should be dosed as doxycycline 200 mg DR tablets once daily for 7 days. This formulation is as effective as the immediate release, however, it is more costly.2 A side-by-side comparison of costs can be seen in Figure 2. When comparing average wholesale prices, the delayed release formulation of doxycycline is approximately 10 times greater in cost compared to immediate release.
Gonorrhea is the second most common STI with an estimate of 1,568,000 new infections each year in the United States.3 It can cause infections in the genitals, rectum, and throat. Gonorrhea is caused by Neisseria gonorrhoeae bacterium with complications such as urethritis with dysuria, urethral pruritus, ectopic pregnancy, infertility and can facilitate transmission of human immunodeficiency virus (HIV).3
In prior CDC 2015 guidelines, the recommended regimen for uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum was ceftriaxone 250 mg IM in a single dose plus azithromycin 1 g orally in a single dose. However, per CDC’s new 2021 update, a single 500 mg IM dose of ceftriaxone is the new recommended regimen. For patients weighing ≥150 kg (300 lbs.), a higher dose is needed, and a single 1 gram IM dose of ceftriaxone should be administered. If ceftriaxone is not available, the alternative regimen is a single 240 mg IM dose of gentamicin plus a single 2 g oral dose of azithromycin or monotherapy of cefixime 800 mg orally in a single dose. If there is a coinfection with Chlamydia trachomatis, the recommended regimen of doxycycline 100 mg twice daily orally for 7 days should be added to the treatment plan. During pregnancy, azithromycin 1 g as a single dose is recommended to treat chlamydia.3
Figure 1: Comparison of gonorrhea and chlamydia recommended treatment regimen recommendations: 2015 vs. 2021 CDC guidelines 1
Figure 2: Average wholesale price of a seven-day chlamydia treatment course of doxycycline delayed release 200mg vs. immediate release 100mg formulations.14
Rationale for Guideline Updates
Several studies have documented the emergence of azithromycin resistance due to its widespread usage to treat infections. For chlamydia treatment, data obtained from azithromycin versus doxycycline studies, show higher treatment failure rates with azithromycin compared to doxycycline.6 A randomized trial conducted by Manavi K, et al, reported 100% treatment success with doxycycline and 75% with azithromycin for the treatment of rectal chlamydia infection.2 Per STI guidelines, Levofloxacin is still recommended as an alternative regimen for chlamydia treatment as it is an effective treatment, but it is more expensive compared to the recommended regimen. Erythromycin is associated with gastrointestinal side effects which excludes it from the alternative treatment regimen options.2
According to a study in 2017, the increasing resistance of azithromycin in treating Neisseria gonorrhoeae threatens dual antimicrobial treatment.7 In this study, the results suggested a direct relation between increasing exposure to azithromycin and higher azithromycin minimum inhibitory concentration (MIC) in Neisseria gonorrhoeae caused by mutations of mtrR, which may result in clinical resistance.7
Over the past 5 years, the percentage of Neisseria gonorrhoeae’s resistance to azithromycin has increased more than 7-fold, from 0.6% in 2013 to 4.6% in 2018.8 On the other hand, ceftriaxone MICs ≥0.125 µg/mL have increased by 0.3% from 2006 to 2011. Further decreased susceptibility to Neisseria gonorrhoeae is expected in the future.9 According to clinical trials, although 250 mg IM ceftriaxone is considered an effective treatment, a higher dose of ceftriaxone is needed as a monotherapy medication for isolates with MICs higher than 0.03 µg/mL. Therefore, 500 mg IM is the new recommended dose for ceftriaxone.10 Gonococcal infection treatment with dual therapy including ceftriaxone and azithromycin has contributed to the emergence of reduced susceptibility to ceftriaxone and created harm to the microbiome and other pathogens.11 Hence, it is beneficial to use an increased dose of ceftriaxone as monotherapy for gonococcal infection treatment. It is recommended to monitor ceftriaxone resistance to prevent treatment failure in the future.3 If a patient has an allergy to cephalosporins, then gentamicin 240 mg IM in a single dose plus azithromycin 2 g orally in a single dose can be used as an alternative regimen. According to a clinical trial, these two medications were effective in treating gonococcal infection.12 Cefixime is considered a cephalosporin alternative therapy if ceftriaxone is not available, or if administration is not feasible. Although cefixime susceptibility to Neisseria gonorrhoeae is decreasing, other oral cephalosporins such as cefpodoxime and cefuroxime are not as efficacious as cefixime.13
Summary
In conclusion, CDC STI guidelines were recently updated in July 2021 and include new recommendations for the treatment of chlamydia and gonorrhea. Be sure to treat chlamydia with a 7-day treatment course of doxycycline 100 mg twice daily (do not use azithromycin 1 g orally in a single dose as the first line). If the patient is infected with uncomplicated gonorrhea treat with an increased dose of ceftriaxone 500 mg IM in a single dose (do not use the 250 mg dose any longer and do not add azithromycin 1 gram to the regimen). With the steady rise of antimicrobial resistance, it is crucial to follow the new recommendations.
References:
- Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections. 2001. http://apps.who.int/iris/bitstream/handle/10665/66818/WHO_HIV_AIDS_2001.02.pdf;jsessionid=4F85802E7CB991B44F314D312E17A3BF?sequence=1. Published 2001. Accessed August 17, 2021.
- STI treatment guidelines. Centers for Disease Control and Prevention. https://www.cdc.gov/std/treatment-guidelines/default.htm. Published 2021. Accessed August 17, 2021.
- Update to cdc’s treatment guidelines for Gonococcal Infection, 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm. Published 2020. Accessed August 17, 2021.
- National overview – sexually transmitted Disease SURVEILLANCE, 2019. Centers for Disease Control and Prevention. https://www.cdc.gov/std/statistics/2019/overview.htm. Published April 13, 2021. Accessed September 2, 2021.
- Carlin E, Flew S. Sexually acquired reactive arthritis. Clinical medicine (London, England). 2016;16(2):193-196. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4952977/. Published 2016.Accessed August 18, 2021.
- Lau A, Bradshaw CS, Lewis D, et al. The efficacy of azithromycin for the treatment of genital mycoplasma genitalium: A systematic review and meta-analysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;61(9):1389-1399. https://pubmed.ncbi.nlm.nih.gov/26240201/. Published November 1,2015. Accessed August 18, 2021.
- Wind CM, de Vries E, Schim van der Loeff MF, et al. Decreased azithromycin susceptibility of neisseria gonorrhoeae isolates in patients recently treated with azithromycin. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017;65(1):37-45. https://pubmed.ncbi.nlm.nih.gov/28510723/. Published July 1, 2017. Accessed August 19, 2021.
- Addressing the Threat of Drug-Resistant Gonorrhea. CDC FACT SHEET. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/drug-resistant-gonorrhea.pdf. Published 2020. Accessed August 29, 2021.
- Bowen V, Braxton J, Davis D, et al. Sexually Transmitted Disease Surveillance 2018. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf. Published 2019.Accessed August 18, 2021.
- Unemo M, Eyre D, Golparian D. Antimicrobial resistance in neisseria gonorrhoeae and treatment of gonorrhea. Methods in molecular biology (Clifton, N.J.). 2019; 1997:37-58 https://pubmed.ncbi.nlm.nih.gov/31119616/. Published May 23, 2019. Accessed August 19, 2021.
- Carnicer-Pont D, Smithson A, Fina-Homar E, et al. First cases of neisseria gonorrhoeae resistant to ceftriaxone in Catalonia, Spain, May 2011. 2012;30(4):218-219. https://www.sciencedirect.com/science/article/abs/pii/S0213005X11003740?via%3Dihub. Published January 14, 2012. Accessed August 19, 2021.
- Linhart Y, Shohat T, Amitai Z, et al. Sexually transmitted infections among brothel-based sex workers in Tel-Aviv area, Israel: High prevalence of pharyngeal gonorrhoea. International journal of STD & AIDS. 2008;19(10):656-659. https://pubmed.ncbi.nlm.nih.gov/18824615/. Published October 19, 2008. Accessed August 19, 2021.
- Singh V, Bala M, Bhargava A, et al. In vitro efficacy of 21 dual antimicrobial combinations comprising novel and currently recommended combinations for treatment of drug resistant gonorrhoea in future era. PloS one. 2018;13(3): e0193678. https://pubmed.ncbi.nlm.nih.gov/29509792/. Published March 6, 2018. Accessed August 19, 2021.
- US Pricing. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at: http://online.lexi.com. Accessed August 18, 2021.